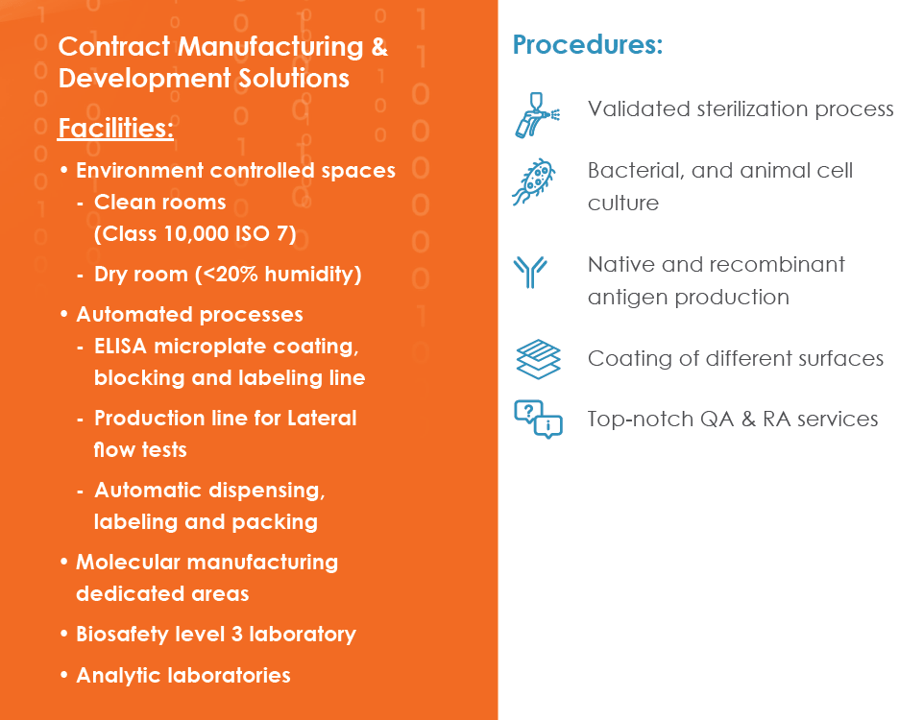
Contract Manufacturing & Development Solutions
Assisting you in transforming your concept into a product
Since its establishment in 1984, Savyon Diagnostics has accumulated extensive experience and broad
know-how in developing and manufacturing of reagents and instrumentation for the clinical diagnostics
community. Building upon our diverse experience in manufacturing, Savyon Diagnostics offers contract
manufacturing and development services to Biotech companies varying from startups to medium and
large enterprises.
We provide high quality products, efficient manufacturing schemes, regulatory affairs proficiency and
dedicated personnel at all levels. Thus, providing the highest level of competence in a cost-effective
manner.
Over the past three decades our skilled team of scientists have developed and transferred to production
a myriad of diagnostic tests. We also offer assay development services, including, prototype design,
scale-up, compilation of SOPs and preparation of technical files for regulatory purposes.
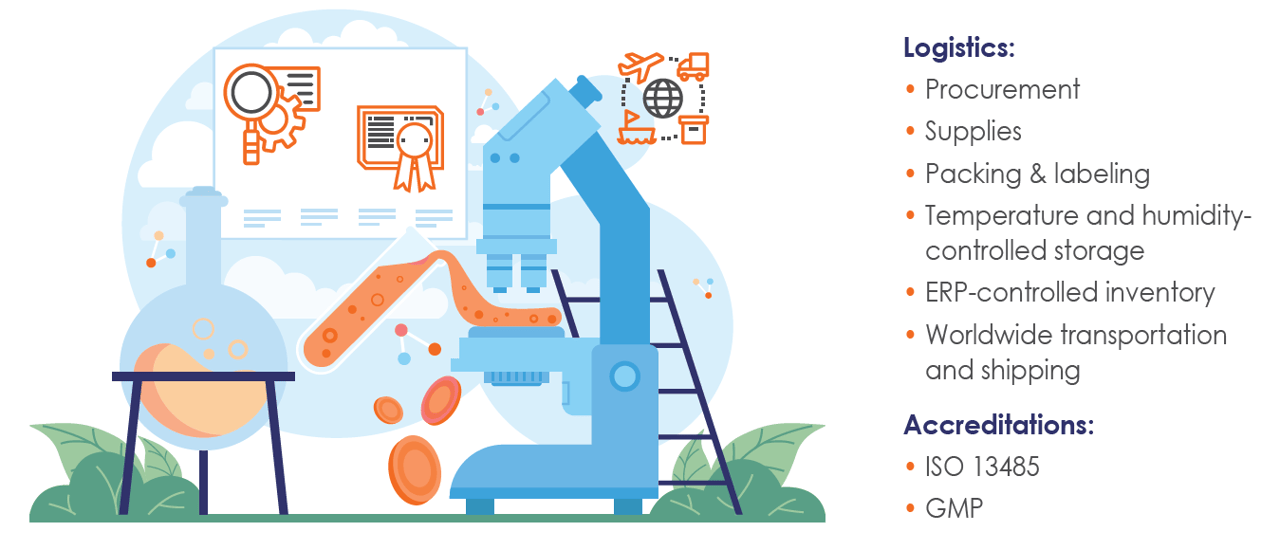
The perfect solution for your diagnostic and biotechnology production
• Quality service
• Technical expertise
• Quick time-to-market by ensuring manufacturability at the development stage of the product
• Custom development and manufacturing
Our Manufacturing capabilities include the following: