Introduction
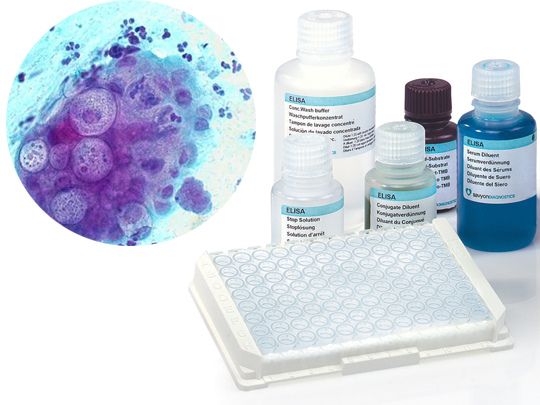
The SeroELISA™ Chlamydia product line is intended for the determination of IgG, IgA and IgM antibodies to Chlamydia in human serum by an Enzyme-Linked Immunosorbent Asssay (ELISA).
The SeroELISA™ Chlamydia test employs the L2 serovar broadly reacting antigen of C. trachomatis. It will detect C. trachomatis, C. psittaci and C. pneumoniae (TWAR) antibodies. Due to the SeroELISA™ pre-calibrated components and assay procedure, the absorbance at 450nm is indicative of IgG, IgA and IgM titer in patient serum specimens.
The SeroELISA™ Chlamydia product line is ideal for high and low volume laboratories
| Catalog No | Product name | Tests/kit | Approvals |
| A111-01 | SeroELISA Chlamydia IgG | 96 | CE 0483/FDA |
| A112-01 | SeroELISA Chlamydia TRUE-IgM | 96 | CE 0483 |
| A113-01 | SeroELISA Chlamydia IgA | 96 | CE0483/FDA |